
Blog
Benefits of Pharmaceutical Track and Trace
The pharmaceutical industry is under immense pressure to ensure patient and drug safety while operating within a complex web of global and national regulations. Still, without effective technology, the supply chain remains vulnerable despite these stringent measures. The World Health Organization's disturbing findings underscore this vulnerability: an estimated 10% of medicines circulating in low and middle-income countries are counterfeit or substandard! This data clearly highlights the urgent need for stronger pharmaceutical tracking systems. By effectively fighting against counterfeit drugs and protecting millions of lives, ensuring patient and drug safety is the primary benefit of implementing such powerful systems.
To clarify, think about the real-life equivalent of this statistic: a family who invests all their money in their child's treatment, unaware that the drugs are substandard or counterfeit. Against these unacceptable situations, many countries have tried to develop pharmaceutical track and trace software and systems as crucial defense lines, tracking the pharmaceutical supply chain from production to end-users.
The real power of comprehensive pharmaceutical track and trace systems lies not only in safeguarding patient and drug safety but also in their vast potential to strengthen public health, prevent economic risks and enhance the overall resilience of healthcare systems. Believing in this potential, we at Tiga Healthcare Technologies have developed a cutting-edge drug traceability system that ensures a clean and transparent supply chain, enhances patient safety and unlocks broader benefits such as supporting rational drug use policies, optimizing recall processes and mitigating economic risks like parallel trade and reimbursement fraud.

Under our DrugXafe brand, Tiga developed and implemented the “End-to-End Pharmaceutical Track and Trace System,” making Türkiye the first country in the world to adopt such a system on a national-scale in 2010. This groundbreaking system was the first system to enable nationwide pharmaceutical traceability. Until that time, traditional pharmaceutical tracking systems only monitored the production process and a few steps in the supply chain, while DrugXafe began tracking and tracing every single medicine box throughout the entire supply chain. This end-to-end approach not only significantly enhanced drug safety but also set a new global standard for pharmaceutical traceability with its additional benefits. Recognizing the success of this approach, the independent auditing and consultancy firm PwC named our pharmaceutical track and trace system a “Best Practice in the Pharma Industry".
In this blog, we’ll delve into the world of pharmaceutical track and trace systems, exploring their benefits and potential before showcasing the unique advantages of DrugXafe.
What is Track and Trace in Pharma?
What is the main aim and main difficulty facing the pharmaceutical supply chain on a global scale? The aim and the challenge are the same: ensuring the reliable supply of drugs to patients. However, a significant challenge hinders this in most countries, which is the threat of illegal activities. Many dangers like counterfeit drugs, reimbursement fraud, smuggling, and parallel trade have the potential to seriously impact patient safety, population health, and the pharma economy. Then, we ask again: what is the track and trace system in pharma? They are the main healthcare IT solutions against these dangers.
In essence, tracking and tracing in the pharma industry is about monitoring the movement of drugs to guarantee drug safety and supply chain transparency efficiently. By serializing each medication box (assigning unique identifiers) and implementing measures like providing ‘shipment and delivery notifications’ for control, these systems provide effective and robust assurance in the ongoing fight against counterfeit drugs.
How Do Pharmaceutical Track and Trace Systems Work?
At the heart of pharmaceutical tracking systems are databases containing critical drug information. Technologies such as barcodes and 2D Data Matrix codes enable the easy creation, scanning and database updating of information related to pharmaceutical products. During the production process, a barcode or 2D Data Matrix containing information specific to the medication is printed on each drug box, making it unique. This ensures that each drug box becomes fully traceable throughout the supply chain.
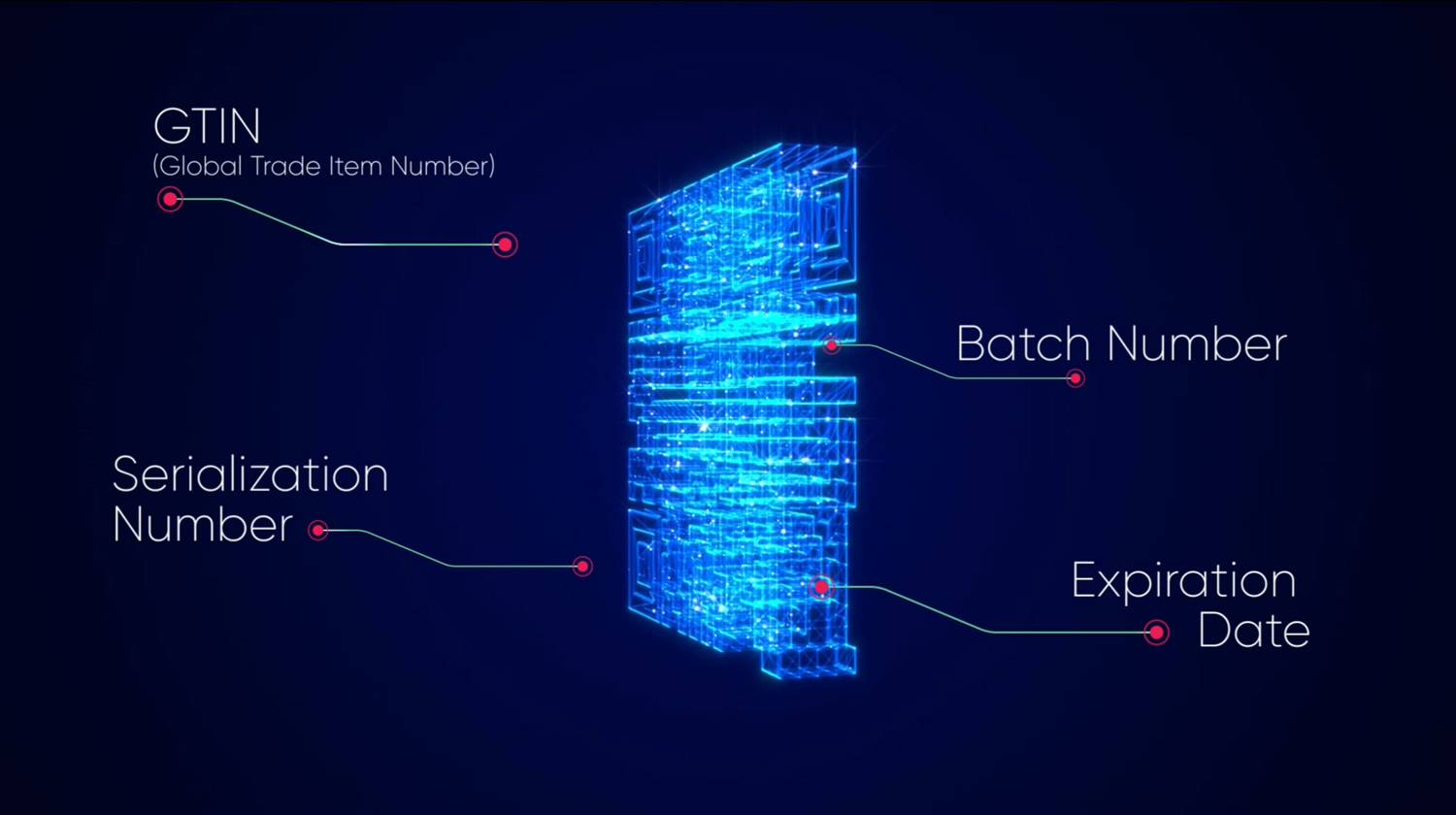
Let's take 2D Data Matrix codes, which we use in DrugXafe, as an example. These codes are indispensable as they compactly encode necessary drug information such as the Global Trade Item Number (GTIN), Expiration Date (XD), Serialization Number (SN) and Batch Number (BN) into a scannable format. Present on every drug in the DrugXafe system; these codes function like mobile libraries, providing stakeholders with the required information.
Each stakeholder in the supply chain scans these codes and sends notifications to the system, indicating the dispatch or receipt of the respective product, thereby ensuring seamless data flow. This data flow and functionality enable precise tracking and verification of each product, eliminating the risk of counterfeits and optimizing inventory management.
This drug data is essential for adhering to rigid regulatory requirements and standards, ensuring drug safety and efficacy. The pharmaceutical industry operates in a zero-tolerance environment governed by a complex web of regional, national and global regulations.

For example, the Drug Supply Chain Security Act (DSCSA) in the United States outlines a standardized approach to identifying and tracking prescription drugs at the package level. Japan's Pharmaceutical Affairs Law mandates regulations for pharmaceuticals, quasi-drugs and medical devices to increase efficacy and safety. As a regional example, the European Union's Falsified Medicines Directive (FMD) imposes strict product serialization and security requirements, including unique identifiers and tamper-evident packaging. Countries with advanced pharmaceutical tracking systems can maintain the highest safety standards to meet these regulatory demands.
As one of the complementary examples of these efforts at the global level, the World Health Organization's Medication Without Harm initiative and related projects see medication traceability as a critical component of patient safety. Addressing medication-related issues as avoidable harm and supporting potent pharmaceutical track and trace systems, the initiative seeks to prevent medication errors, counterfeit drugs and adverse drug reactions. Focusing on traceability aims to enhance patient outcomes and create a safer global healthcare environment. Then, the next question is, if implementing pharmaceutical tracking systems is almost mandatory, what are the technical and operational challenges in fulfilling this obligation?

Pharmaceutical Track and Trace Challenges
The rewards of these systems are substantial but so are the challenges. There are considerable obstacles to implementing and maintaining effective pharmaceutical tracking systems. The intricate nature of global supply chains and a patchwork of heavy regulations create a challenging environment.
Overcoming barriers to developing required technologies that seamlessly integrate various stakeholders requires addressing several issues. For instance, ensuring smooth interoperability between different systems while protecting sensitive drug data is crucial. Let’s explore the main challenges step-by-step.
Supply Chain Complexity
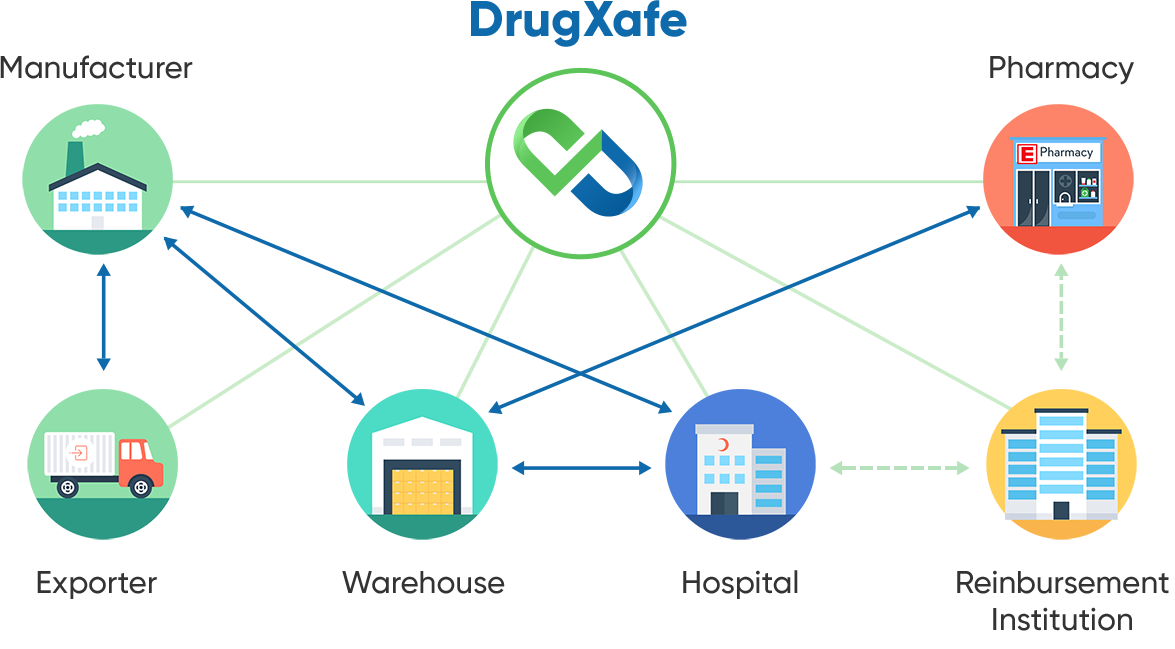
The complexity of pharmaceutical supply chains in this global era exemplifies a significant challenge in tracking and tracing drugs. With multiple stakeholders, including manufacturers, wholesalers, distributors, retailers and patients, various links introduce changing regulatory, logistical, data management and safety issues.
Each link in the chain adds a layer of complexity and complicates achieving real-time visibility and coordination. It's essential to ensure accurate and consistent tracking of drugs in a comprehensive way that integrates diverse data sources and streamlines communication across all parties involved. Addressing this complexity is crucial for maintaining the integrity and safety of the pharmaceutical supply chain.
Interoperability Issues
Interoperability in pharmaceutical tracking systems presents a significant challenge, primarily due to the diverse data standards and technologies employed during serialization processes. Discrepancies between national and international systems can create gaps in traceability and disrupt data sharing, making it difficult for all stakeholders in the supply chain to collaborate effectively. These challenges complicate operations and hinder the creation of a seamless, transparent tracking environment.
The technological dimension is the most critical component of achieving interoperability. A comprehensive pharmaceutical tracking solution must interoperate various systems within the healthcare and pharmaceutical ecosystem—such as reimbursement institutions, insurance companies, pharmacy and hospital software and identification and terminology systems. By strengthening data infrastructures and implementing effective integrations, these systems can achieve seamless communication, ensuring an efficient and interconnected framework across all stakeholders.
Security & Privacy Concerns
Pharmaceutical tracking systems should be designed to be highly secure to ensure drug safety and supply chain integrity. Let's take barcode technology, for instance. Advanced tracking technologies based on serialization with 2D Data Matrix codes provide unparalleled protection against counterfeit drugs.
Beyond counterfeit prevention, these systems must also prioritize data privacy. Ensuring the confidentiality of patient information is a cornerstone of ethical pharmaceutical practices. Robust encryption, secure data transmission, and strict access controls are essential to safeguard sensitive information. By addressing both security and privacy concerns, pharmaceutical tracking systems can build trust among stakeholders while enhancing the overall resilience of the supply chain.

Technological Barriers
Pharmaceutical tracking systems rely on advanced technologies like serialization, secure data management and real-time data integration, which, while highly effective, can be resource-intensive to implement. Integrating these technologies across diverse platforms and stakeholders introduces compatibility challenges that can hinder seamless operation. Without resolving these issues, technological obstacles can result in delays, inflated costs and gaps in traceability. To overcome these barriers, scalable and interoperable solutions must be prioritized, alongside equipping all stakeholders in the supply chain with the required technology and expertise.
Stakeholder Resistance
Another operational barrier is stakeholder resistance against track and trace pharmaceuticals. Various stakeholders may need help adopting new technologies due to concerns about costs, troubles with existing processes or the complexity of integration. This resistance can stem from a reluctance to change or fear of functional impacts, leading to delays and inconsistencies in system implementation.
One of the most critical elements in facilitating stakeholder participation is the proactive role and contribution of regulatory bodies and public authorities. Their approach to the issue, combined with their contributions and potential implementation of control mechanisms, significantly influences overall participation. Therefore, early engagement of stakeholders in the transition process for these changes is crucial, as well as providing clear benefits of the new system and offering support, training and time to facilitate a smooth transition.
Now is the perfect time to examine DrugXafe, which offers specific solutions to each of these challenges, implementation strategies, security measures and an inclusive approach!
![]()
Tiga's Global Role in Pharmaceutical Tracking System
We launched DrugXafe in 2010 for the Turkish Ministry of Health, a major step for the track and trace solutions for the pharmaceutical supply chain. As the world's first national-scale end-to-end pharmaceutical tracking system, DrugXafe has radically transformed drug tracking and control by offering complete visibility and real-time data at the item/box level with unique 2D Data Matrix codes. This innovation earned DrugXafe two United Nations - WSIS awards for its successful implementation in both Türkiye and Saudi Arabia.
DrugXafe actively operates across three countries, serving over 65,000 stakeholders, such as pharmacies, consumption centers, wholesalers, manufacturers, exporters and reimbursement institutions. With a track history of handling more than 35 billion pharmaceuticals, we continue to expand our reach globally. Let's explain how we reached this!
2D Data Matrix Codes for Product Serialization
DrugXafe establishes a comprehensive digital record for each drug box using 2D Data Matrix codes for serialization. These codes are two-dimensional barcodes that store information in both vertical and horizontal dimensions, allowing for a higher data density. They are highly compact and can encode a significant amount of data in a small space, making them ideal for applications where space is limited, such as labeling pharmaceuticals. In DrugXafe, these codes include detailed drug information like Global Trade Item Number (GTIN), Serialization Number (SN), Expiration Date (XD) and Batch Number (BN) for consistent national-level tracking and tracing. Then, what are the real-life results of serialization with these efficient codes?
DrugXafe prevents unauthorized (counterfeit or substandard) drugs without 2D Data Matrix codes from entering the national supply chain thanks to the application of these barcodes at the production stage. The system ensures patient safety by verifying drugs before they reach patients and blocking expired products from being dispensed. Additionally, it supports processes such as Rational Drug Use and Drug Recall by operating the ecosystem based on rule sets established for pharmaceutical policies defined by public authorities and regulators. With these features, DrugXafe enables the pharmaceutical supply chain to be managed transparently and effectively. Now, let’s delve deeper into these advantages.
Benefits of DrugXafe
y ensuring authenticity verification and comprehensive track and trace capabilities, DrugXafe revolutionizes drug safety with its secure and effective approach. Let’s get into the details!

Blocking Counterfeit and Substandard Drugs
Serialization via 2D Data Matrix codes is a guaranteed way of meticulously tracking each drug's path from production and importation to patient delivery. Every drug has a unique digital fingerprint, from production and importation to patients' medicine cabinets.
This secure system is like a defense line, blocking counterfeit and substandard drugs and ensuring only genuine medication reaches patients. DrugXafe plays a crucial role in protecting the health of the population.
Reimbursement & Insurance Processes Enhancement
As we said, every drug gets its own unique digital ID or, in other words, a barcode that's usable only once. This means no more double reimbursements. DrugXafe tracks the sale of drugs to patients, ensuring that reimbursement and insurance payment processes are also kept under control while also building a wall against reimbursement fraud by tracking production, sales, costs and returns. For insurance companies and reimbursement institutions, the ability to digitally verify every step is a key advantage, making the process smoother and safer for everyone involved.
Effective Stock and Inventory Management
With DrugXafe, stock management becomes a simple and seamless process. Its real-time tracking system provides comprehensive visibility of drug inventory for all stakeholders in the supply chain, including manufacturers, warehouses, pharmacies, etc. Stakeholders can easily track how much of each drug they have, where these drugs are located and when they need to restock. This eliminates critical issues such as running out of essential drugs or dealing with expired stock. By enabling effective stock and supply management, DrugXafe ensures that patients receive the medications they need when they need them.
Drug Recall Management
If a drug is recalled due to safety concerns, DrugXafe springs into action with precision and efficiency. Whether it's a faulty batch, an expired product or unexpected side effects, the system swiftly identifies the affected drugs and initiates their removal process from the market. By providing real-time tracking and detailed data, it helps stakeholders trace and withdraw the recalled products at every level of the supply chain. This minimizes risks, prevents further distribution of recalled drugs and ensures that only safe and effective drugs reach patients, safeguarding public health.
Data Management and Healthcare Insights
DrugXafe isn't just about tracking drugs; it’s about transforming healthcare by turning data into powerful insights. The system offers valuable data for healthcare authorities and managers to create detailed analyses, reports, policy recommendations and insights. There are also more gains, such as disease maps, predicting drug shortages and developing fast responses to pandemics. Additionally, it supports the implementation of drug administration, guidelines and policies such as Rational Drug Use, defined by pharmaceutical authorities, particularly for antibiotics and other sensitive drug groups. It gives health authorities the tools to build a smarter, stronger and more resilient healthcare system.

Tracking and Tracing of Specific Drugs for Enhanced Safety
DrugXafe provides a responsible control mechanism to enhance patient safety and ensure the proper use of sensitive and controlled drug groups, such as narcotics and psychotherapeutics. By comprehensively monitoring the pharmaceutical supply chain and utilizing the robust database created from real-time data, the system enables detailed tracking and tracing of these high-risk medications.
Central e-Prescription integration adds another layer of security by monitoring prescription patterns and issuing real-time alerts for unusual activities. This approach minimizes the risk of misuse or abuse, ensuring that these drugs are used strictly in accordance with healthcare policies and regulations. DrugXafe thus plays a critical role in safeguarding public health and maintaining the integrity of controlled drug management.
The pharmaceutical industry is undergoing significant changes driven by technological advancements and emerging trends. We lead in this manner by updating DrugXafe and our Drug Traceability product family. DrugXafe Mobile App enables patients to check their drugs' status by scanning the 2D Data Matrix or entering drug identifiers manually. This way, patients can access information about expiration dates, recall information, drug prices, adverse reaction notifications, announcements and the nearest pharmacies in seconds.
The Aggregation Management System, which complements the Pharmaceutical Track and Trace System at the supply chain layer and is designed to track and monitor drug movements at the box-package-pallet scale, ensures the accurate grouping of drugs. This system facilitates the tracking of drugs, particularly at stages involving bulk movements such as production, import, export, and warehouse operations. As an integral part of DrugXafe, it strengthens the platform’s ability to track pharmaceuticals comprehensively, ensuring a seamless flow of information across all supply chain layers. This enhancement not only improves operational efficiency but also supports the broader goal of ensuring drug safety and supply chain transparency.
In summary, effective pharmaceutical tracking and tracing systems are critical for combating counterfeit drugs, ensuring patient safety and enhancing efficiency in the pharmaceutical industry. Currently active in three countries worldwide and recognized twice as an 'e-Health Champion' by the United Nations WSIS, DrugXafe has proven itself as a reliable and effective solution in the global pharmaceutical ecosystem. By enabling national-scale, box-level tracking of the supply chain, optimizing inventory management and ensuring regulatory compliance, DrugXafe addresses key drug safety challenges while creating a transparent, reliable and efficiently managed supply chain.
Let’s build a safer pharmaceutical future together with innovative tracking and tracing technologies!









